Research focuses in the Drug Discovery and Delivery Laboratory:
Cell-specific and multifunctional drug delivery in vivo has been regarded as one of the most challenging issues in the field of drug delivery. A wide variety of cell types in humans still cannot be efficiently and specifically reached by delivery systems such as lung epithelial cells, metastatic tumor cells, and immune cells. An even more formidable task is delivering multiple payloads into specific cells and tissues. To address these challenges, my laboratory-Drug Discovery and Delivery Laboratory focuses on the following research areas: 1) to develop cell specific drug delivery systems; 2) to construct multifunctional drug delivery systems; 3) to demonstrate therapeutic efficacy of these systems in animal models for treating genetic disorders, infectious diseases, as well as cancers.
Platform biotechnologies under development in the Drug Discovery and Delivery Laboratory:
- Developing new biomaterials such as lipid nanoparticles for therapeutic and diagnostic applications
- Engineering RNA molecules including mRNAs and the CRISPR systems
- Constructing adoptive cell therapy such as macrophages and stem cells
LIPID NANOPARTICLES
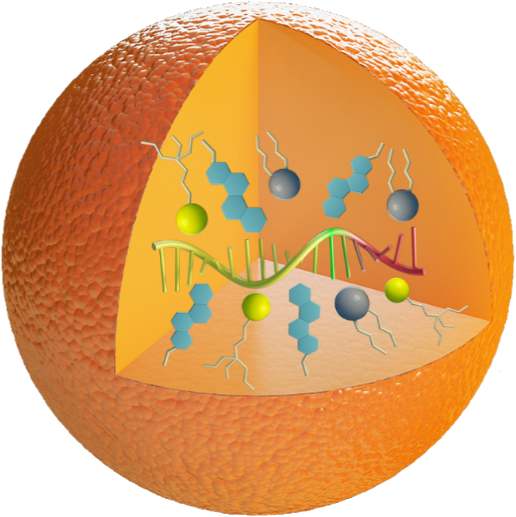
Nanomaterials have shown great promise for a wide variety of applications. For example, lipid and lipid-derived nanoparticles are able to encapsulate multiple types of agents and achieve multifunctions. We have invented numerous classes of novel of lipid-derived or lipid-like nanoparticles (LNPs and LLNs), which demonstrates improved delivery efficiency of mRNA and the CRISPR systems. For example, TT3 LLNs restore the human factor IX (hFIX) level to normal physiological values in FIX-knockout mice. Also, TT3 LLNs as multifunctional oncolytic nanoparticles carrying therapeutic self replicating RNA can eliminate established tumors and prime systemic immunity. Additionally, our functionalized lipid-like nanoparticles have demonstrated potent base editing capability in vivo at low doses. Notably, the dose-response suggests an EC50 of <0.125 mg/kg, a potency that is unmatched in previous reports of in vivo base editing. Consequently, these nanomaterials merit further development for therapeutic applications.
RNA ENGINEERING
Poly-ribonucleic acids (RNAs) play crucial roles in living organisms. Now, not only has RNA been discovered for new functions in biology, but it also has potential to emerge as an essential class of therapeutic medicines like small-molecule and protein drugs. We have applied an engineering approach such as structural alteration and chemical modifications to regulate the functions of diverse RNAs. For example, through a comprehensive analysis of endogenous gene expression (4248 mRNAs) and de novo design of UTRs, we have identified the optimal combination of 5’ and 3’ UTR, termed as NASAR. Importantly, NASAR mRNAs delivered by lipid-derived nanoparticles show a dramatic expression of potential SARS-CoV-2 antigens and induce potent antigen-specific antibodies in vaccinated mice. Engineered RNAs such as mRNAs and the CRISPR RNA facilitate a broad range of therapeutic applications.
CELL THERAPY
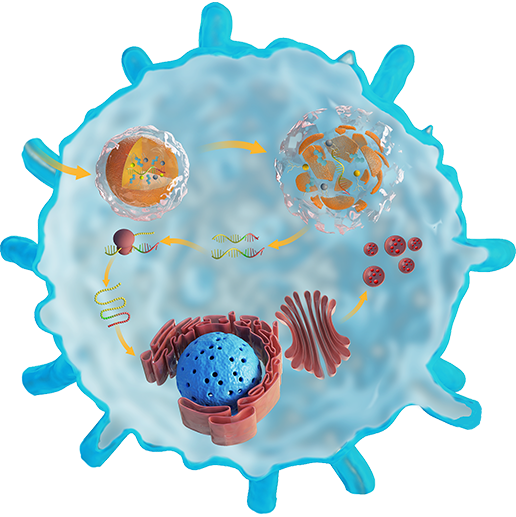
Cell therapy has revolutionized the treatment of deadly diseases such as certain types of blood cancers. More importantly, this strategy can be applied to diverse diseases. For example, we have developed macrophages containing antimicrobial peptides linked to cathepsin B in the lysosomes (MACs) via the vitamin C lipid nanoparticles-mediated delivery of mRNA. Our results demonstrate that adoptive MAC transfer leads to the elimination of MDR bacteria, including S. aureus and E. coli, and the complete recovery of immunocompromised septic mice. Our work provides a new strategy for overcoming multidrug-resistant (MDR) bacteria-induced sepsis and opens up possibilities for nanoparticle-enabled cell therapy for various diseases such as bacterial infections and cancers.
